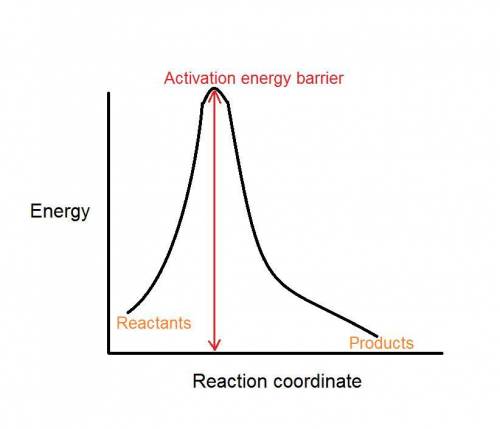
What happens as the activation energy increases?
the pressure of the system decreases.
...


Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1


Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Questions

Mathematics, 25.07.2019 05:00


Computers and Technology, 25.07.2019 05:00


English, 25.07.2019 05:00



Health, 25.07.2019 05:00


Mathematics, 25.07.2019 05:00




Biology, 25.07.2019 05:00




Computers and Technology, 25.07.2019 05:00

Mathematics, 25.07.2019 05:00