Chemistry, 29.10.2019 07:31 acavalieri72
Consider the dissolution of 2.50 grams of salt xy in 75.0 ml of water within a calorimeter. the temperature of the water decreased by 0.93 oc. the heat capacity of the calorimeter is 42.2 j/oc. the density of the water (and the solution) is 1.00 g/ml. the specific heat capacity of the solution is 4.184 j/goc. calculate the enthalpy change for dissolving this salt on a energy per mass basis (units of j/g).

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1

Chemistry, 23.06.2019 09:10
A155.0 9 piece of copper at 182 °c is dropped into 2500 g of water at 23.9 °c. (the specific heat of copper is 0.385 1/9°c.) calculate the final temperature of the mixture. (assume no heat loss to the surroundings.)
Answers: 2

Chemistry, 23.06.2019 11:30
Which of the following is the most likeley example of an favorable mutation a. a mutation that makes a rabbit able run faster b. a mutation that changes the rabbit's fur to bright orange c. a mutation that changes the color of the rabbit's eyes d. a mutation that gives a rabbit a third ear
Answers: 1

Chemistry, 23.06.2019 14:30
2.38g of black copper (ii) oxide is completely reduced by hydrogen to give copper and water. what are the masses of copper and water formed? ?
Answers: 1
You know the right answer?
Consider the dissolution of 2.50 grams of salt xy in 75.0 ml of water within a calorimeter. the temp...
Questions

Mathematics, 28.06.2020 16:01

Mathematics, 28.06.2020 16:01






Biology, 28.06.2020 16:01

Chemistry, 28.06.2020 16:01

English, 28.06.2020 16:01



Mathematics, 28.06.2020 16:01

Social Studies, 28.06.2020 16:01

English, 28.06.2020 16:01


English, 28.06.2020 16:01



Mathematics, 28.06.2020 16:01

![q=[q_1+q_2]](/tpl/images/0350/9166/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0350/9166/1d50b.png)
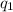 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter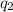 = heat absorbed by the water
= heat absorbed by the water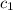 = specific heat of calorimeter =
= specific heat of calorimeter = 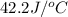
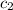 = specific heat of water =
= specific heat of water = 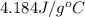
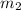 = mass of water =
= mass of water = 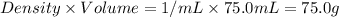
 = change in temperature =
= change in temperature = 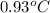
![q=[(42.2J/^oC\times 0.93^oC)+(75.0g\times 4.184J/g^oC\times 0.93^oC)]](/tpl/images/0350/9166/57473.png)
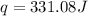
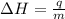
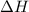 = enthalpy change = ?
= enthalpy change = ?