
Chemistry, 29.10.2019 19:31 yasameenakbari
From the calorimetric data, calculate δh for the reaction that occurs on mixing. assume that the calorimeter absorbs only a negligible quantity of heat, that the total volume of the solution is 100.0 ml, and that the specific heat and density of the solution after mixing are the same as that of pure water.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1


Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
From the calorimetric data, calculate δh for the reaction that occurs on mixing. assume that the cal...
Questions



Mathematics, 18.06.2021 22:00

Mathematics, 18.06.2021 22:00



English, 18.06.2021 22:00



Mathematics, 18.06.2021 22:00

English, 18.06.2021 22:00






Mathematics, 18.06.2021 22:00

English, 18.06.2021 22:00


Business, 18.06.2021 22:00

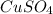 is 1.0 M and its volume is 50.0 ml or
is 1.0 M and its volume is 50.0 ml or 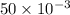 L (as 1 L = 1000 mL).
L (as 1 L = 1000 mL).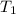 =
= 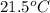 and
and 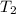 =
= 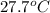
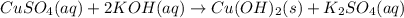
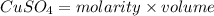
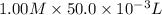
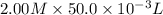
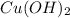 formed = moles
formed = moles 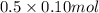
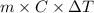
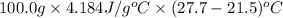
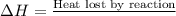
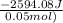
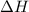 for the given reaction that occurs on mixing is -51.9 kJ/mol.
for the given reaction that occurs on mixing is -51.9 kJ/mol. 

