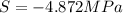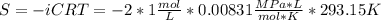Chemistry, 29.10.2019 21:31 tynyiaawrightt
Assuming that the nacl is completely ionized, calculate how much it will lower the solute potential of the soil at 20°c using the solute potential equation: ѱs = –icrt where i is the ionization constant (2 for nacl), c is the molar concentration (in mol/l), r is the pressure constant [r = 0.00831 l • mpa/(mol • k)], and t is the temperature in kelvin (273 + °c). how much will the solute potential of the soil be lowered at 20°c?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 23.06.2019 17:30
Alithium atom has three protons, three neutrons, and three electrons. what is the overall charge on this atom?
Answers: 1

Chemistry, 23.06.2019 21:30
Listed below are the amounts of mercury (in parts per million, or ppm) found in tuna sushi sampled at different stores. the sample mean is 0.836 ppm and the sample standard deviation is 0.253 ppm. use technology to construct a 90% confidence interval estimate of the mean amount of mercury in the population.
Answers: 2
You know the right answer?
Assuming that the nacl is completely ionized, calculate how much it will lower the solute potential...
Questions


History, 03.04.2020 22:06






Biology, 03.04.2020 22:07




Mathematics, 03.04.2020 22:07



Mathematics, 03.04.2020 22:07


Biology, 03.04.2020 22:07