
Chemistry, 29.10.2019 21:31 Adolfosbaby
A0.5222 g solid sample containing a mixture of lacl3 (molar mass = 245.26 g/mol) and ce(no3)3 (molar mass = 326.13 g/mol) was dissolved in water. the solution was titrated with kio3 , producing the precipitates la(io3)3 and ce(io3)3 . for the complete titration of both la3+ and ce3+ , 42.81 ml of 0.1250 m kio3 was required. calculate the mass fraction of la and ce in the sample.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
A0.5222 g solid sample containing a mixture of lacl3 (molar mass = 245.26 g/mol) and ce(no3)3 (molar...
Questions

Mathematics, 03.05.2020 13:28

Mathematics, 03.05.2020 13:28

History, 03.05.2020 13:28

English, 03.05.2020 13:28

Mathematics, 03.05.2020 13:28


Computers and Technology, 03.05.2020 13:28

Mathematics, 03.05.2020 13:28




Mathematics, 03.05.2020 13:28



Mathematics, 03.05.2020 13:28

History, 03.05.2020 13:28

Mathematics, 03.05.2020 13:28

Mathematics, 03.05.2020 13:28


Mathematics, 03.05.2020 13:29

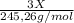 +
+ 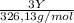 (1)
(1)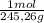 ×
×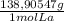 = 0,1022g of La
= 0,1022g of La 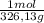 ×
×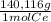 = 0,1468g of Ce
= 0,1468g of Ce 


