
Ammonia (nh3) ionizes according to the following reaction: nh3(aq) + h2o(l) ⇌ nh4+(aq) + oh–(aq) the base dissociation constant for ammonia (nh3) is kb = 1.8 × 10–5. ammonia (nh3) also has a chloride salt, ammonium chloride (nh4cl), which is soluble in water. if 0.070 m of ammonia (nh3) and 0.035 m of its salt ammonium chloride (nh4cl) are mixed in a solution, what is the ph of this solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Ammonia (nh3) ionizes according to the following reaction: nh3(aq) + h2o(l) ⇌ nh4+(aq) + oh–(aq) th...
Questions


Biology, 29.01.2020 12:00




Mathematics, 29.01.2020 12:00


Mathematics, 29.01.2020 12:00



Computers and Technology, 29.01.2020 12:00



History, 29.01.2020 12:00

Chemistry, 29.01.2020 12:00

History, 29.01.2020 12:00


Mathematics, 29.01.2020 12:01

Mathematics, 29.01.2020 12:01

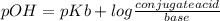
![pOH = pKb + log\frac{[NH_{4}^{+} ]}{[NH_{3}]} =4.7+log\frac{0.035M}{0.070M} =4.4](/tpl/images/0351/7427/0637f.png)



