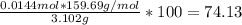Asample weighing 3.102 g is a mixture of fe2o3 (molar mass = 159.69 g/mol) and al2o3 (molar mass = 101.96 g/mol). when heat and a stream of h2 gas is applied to the sample, the fe2o3 reacts to form metallic fe and h2o(g). the al2o3 does not react. if the sample residue (the solid species remaining after the reaction) weighs 2.413 g, what is the mass fraction of fe2o3 in the original sample?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1
You know the right answer?
Asample weighing 3.102 g is a mixture of fe2o3 (molar mass = 159.69 g/mol) and al2o3 (molar mass = 1...
Questions


Mathematics, 30.04.2021 19:20



English, 30.04.2021 19:20




English, 30.04.2021 19:20

Mathematics, 30.04.2021 19:20


Mathematics, 30.04.2021 19:20

Mathematics, 30.04.2021 19:20


Mathematics, 30.04.2021 19:20

Chemistry, 30.04.2021 19:20


Mathematics, 30.04.2021 19:20