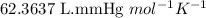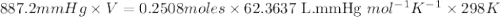Chemistry, 30.10.2019 01:31 scottmichetti
When solid calcium carbonate is reacted with aqueous hydrochloric acid, the products of the reaction include aqueous calcium chloride, liquid water, and gaseous carbon dioxide. calculate the volume of co₂ gas collected over water at 25.0 °c when 25.1 g of calcium carbonate is added to excess hydrochloric acid if the total pressure is 911 mm hg. the vapor pressure of water at 25.0 °c is 23.8 mm hg.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
When solid calcium carbonate is reacted with aqueous hydrochloric acid, the products of the reaction...
Questions



Computers and Technology, 23.08.2019 00:10


Computers and Technology, 23.08.2019 00:10








Computers and Technology, 23.08.2019 00:10


Computers and Technology, 23.08.2019 00:10





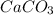 = 100.0869 g/mol
= 100.0869 g/mol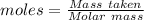
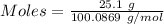
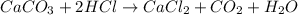
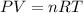
![25^oC=[25+273]K=298K](/tpl/images/0351/9682/df1f6.png)