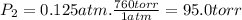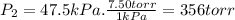Chemistry, 30.10.2019 01:31 alexmoy45p8yd7v
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of the specified gases. bulb a: 150. ml of co(g) at 190. torr bulb b: 300. ml of ar(g) at 0.500 atm bulb c: 750. ml of kr(g) at 75.994 kpa 1. after both stopcocks are opened and the gases allowed to diffuse throughout, what will be the ultimate total pressure? 2. what is the partial pressure of co(g)?
3. what is the mole fraction of co₂(g)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1
You know the right answer?
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of t...
Questions

English, 29.09.2020 14:01

History, 29.09.2020 14:01


Mathematics, 29.09.2020 14:01

Chemistry, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01





Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01




History, 29.09.2020 14:01

Social Studies, 29.09.2020 14:01



Social Studies, 29.09.2020 14:01