
Chemistry, 30.10.2019 01:31 musfirahkhurram
Many common weak bases are derivatives of nh3, where one or more of the hydrogen atoms have been replaced by another substituent. such reactions can be generically symbolized as nx3(aq)+h2o(l)⇌hnx3+(aq)+oh−(aq) where nx3 is the base and hnx3+ is the conjugate acid. the equilibrium-constant expression for this reaction is kb=[hnx3+][oh−][nx3] where kb is the base ionization constant. the extent of ionization, and thus the strength of the base, increases as the value of kb increases. ka and kb are related through the equation ka×kb=kw as the strength of an acid increases, its ka value increase and the strength of the conjugate base decreases (smaller kb value). part a if kb for nx3 is 9.0×10−6, what is the poh of a 0.175 m aqueous solution of nx3? express your answer numerically.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3


Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
Many common weak bases are derivatives of nh3, where one or more of the hydrogen atoms have been rep...
Questions

English, 06.01.2021 03:30

Mathematics, 06.01.2021 03:30

Mathematics, 06.01.2021 03:30

Biology, 06.01.2021 03:30



Mathematics, 06.01.2021 03:30

Business, 06.01.2021 03:30

Mathematics, 06.01.2021 03:30






English, 06.01.2021 03:30

Mathematics, 06.01.2021 03:30


Social Studies, 06.01.2021 03:30


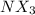 .
.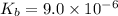
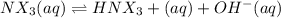
![K_b=\frac{[HNX_3][OH^-]}{[NX_3]}](/tpl/images/0351/9348/b0976.png)
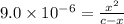
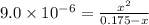
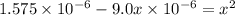
![[HNX_3]=[OH^-]=0.0012505 M](/tpl/images/0351/9348/339f9.png)
![pOH=-\log[OH^-]](/tpl/images/0351/9348/fe336.png)
![pOH=-\log[0.0012505 ]=2.90](/tpl/images/0351/9348/32d39.png)


