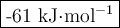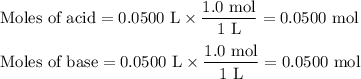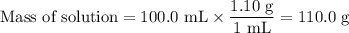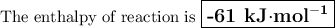Chemistry, 30.10.2019 01:31 jmwmarshall
3. during an experiment where 50.0 ml of a 1.0 m acid solution was mixed with 50.0 ml of a 1.0 m base solution, the temperature change was measured to be 6.5 oc. if the density of the resulting mixture is 1.10 g/ml, specific heat of the solution is 4.18 j/goc, and the cal constant was 12.0 j/oc, what is the hrxn in kj/mol acid?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
3. during an experiment where 50.0 ml of a 1.0 m acid solution was mixed with 50.0 ml of a 1.0 m bas...
Questions


Mathematics, 20.11.2020 04:30


Mathematics, 20.11.2020 04:30


Computers and Technology, 20.11.2020 04:30

Spanish, 20.11.2020 04:30

Mathematics, 20.11.2020 04:30




Physics, 20.11.2020 04:30



English, 20.11.2020 04:30


Arts, 20.11.2020 04:30

Mathematics, 20.11.2020 04:30

English, 20.11.2020 04:30

Mathematics, 20.11.2020 04:30