
Lemon juice has a ph of about 2.5. assuming that the acidity of lemon juice is due solely to citric acid, that citric acid is a monoprotic acid, and that the density of lemon juice is 1.0 g/ml, then the citric acid concentration calculates to 0.5% by mass. estimate the volume of 0.0100 m naoh required to neutralize a 3.71-g sample of lemon juice. the molar mass of citric acid is 190.12 g/mol.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
Lemon juice has a ph of about 2.5. assuming that the acidity of lemon juice is due solely to citric...
Questions

History, 26.09.2019 01:30

Biology, 26.09.2019 01:30

Mathematics, 26.09.2019 01:30



Biology, 26.09.2019 01:30





SAT, 26.09.2019 01:30



Advanced Placement (AP), 26.09.2019 01:30


Mathematics, 26.09.2019 01:30

Mathematics, 26.09.2019 01:30


Physics, 26.09.2019 01:30

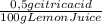 = 0,01855 g of citric acid. In moles:
= 0,01855 g of citric acid. In moles: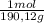 = 9,76x10⁻⁵ moles of citric acid.
= 9,76x10⁻⁵ moles of citric acid.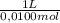 = 9,76x10⁻³L ≡ 9,76 mL
= 9,76x10⁻³L ≡ 9,76 mL

