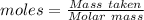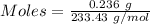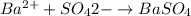Chemistry, 30.10.2019 03:31 putaprincess16
Asolution contains an unknown mass of dissolved barium ions. when sodium sulfate is added to the solution, a white precipitate forms. the precipitate is filtered and dried and then found to have a mass of 236 mg. what mass of barium was in the original solution? (assume that all of the barium was precipitated out of solution by the reaction.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1
You know the right answer?
Asolution contains an unknown mass of dissolved barium ions. when sodium sulfate is added to the sol...
Questions

Biology, 18.07.2019 02:30

Advanced Placement (AP), 18.07.2019 02:30

Health, 18.07.2019 02:30



Mathematics, 18.07.2019 02:30

Mathematics, 18.07.2019 02:30


Mathematics, 18.07.2019 02:30


Mathematics, 18.07.2019 02:30


Mathematics, 18.07.2019 02:30


Social Studies, 18.07.2019 02:30




Engineering, 18.07.2019 02:30

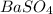 obtained on precipitation = 236 mg
obtained on precipitation = 236 mg