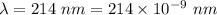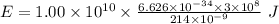Chemistry, 30.10.2019 03:31 lilyella1004
Part a the emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if there are 1.00×1010 atoms of zinc emitting light in the instrument flame at any given instant, what energy (in joules) must the flame continuously supply to achieve this level of emission? express your answer numerically in joules.
part b during an emission, electrons move from a higher energy orbital to a lower energy orbital. which of the following are valid transitions that produce lines in the emission spectrum of zn?
check all that apply.
1-[ar]4s13d106s1→[ar]4s23d10
2-[ar]4s23d10→[ar]4s23d104p2
3-[ar]4s23d10→[ar]3d10
4-[ar]4s23d10→[ar]4s13d11
5-[ar]3d10→[ar]4s23d10
6-[ar]4s23d10→[ar]4s13d106s1

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
Part a the emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if...
Questions


Mathematics, 11.03.2021 18:10

Biology, 11.03.2021 18:10

Mathematics, 11.03.2021 18:10

English, 11.03.2021 18:10

English, 11.03.2021 18:10

Mathematics, 11.03.2021 18:10


Business, 11.03.2021 18:10


Mathematics, 11.03.2021 18:10



Mathematics, 11.03.2021 18:10



History, 11.03.2021 18:10

Chemistry, 11.03.2021 18:10

Social Studies, 11.03.2021 18:10

Mathematics, 11.03.2021 18:10

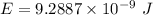
![[Ar]4s^13d^{10}6s^1\rightarrow [Ar]4s^23d^{10}](/tpl/images/0352/1669/c1431.png)
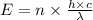
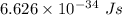
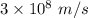
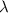 is the wavelength
is the wavelength