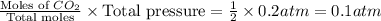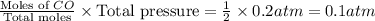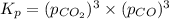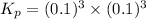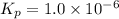When heated, lanthanum(iii) oxalate decomposes as follows: la2(c2o4)3(s) < ===> la2o3(s) + 3 co2(g) + 3 co(g) starting with just the oxalate in a 10.0 l flask, at equilibrium the total pressure observed is 0.200 atm. what is the value of kp for the equilibrium? (dalton’s law of partial pressure! )

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
When heated, lanthanum(iii) oxalate decomposes as follows: la2(c2o4)3(s) < ===> la2o3(s) + 3...
Questions

Mathematics, 20.01.2020 01:31

Social Studies, 20.01.2020 01:31


Mathematics, 20.01.2020 01:31

Biology, 20.01.2020 01:31

History, 20.01.2020 01:31

Computers and Technology, 20.01.2020 01:31

Mathematics, 20.01.2020 01:31


Mathematics, 20.01.2020 01:31




Chemistry, 20.01.2020 01:31

History, 20.01.2020 01:31

Chemistry, 20.01.2020 01:31

Mathematics, 20.01.2020 01:31


English, 20.01.2020 01:31

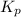 for the equilibrium is
for the equilibrium is 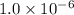
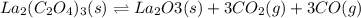
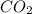 and
and 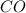 .
.