
Chemistry, 30.10.2019 05:31 steven123pink
A2.137 g sample of a solid mixture containing only potassium carbonate ( mm=138.2058 g/mol ) and potassium bicarbonate ( mm=100.1154 g/mol ) is dissolved in distilled water. a volume of 31.70 ml of a 0.747 m hcl standard solution is required to titrate the mixture to a bromocresol green end point. calculate the weight percent of potassium carbonate and potassium bicarbonate in the mixture.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

You know the right answer?
A2.137 g sample of a solid mixture containing only potassium carbonate ( mm=138.2058 g/mol ) and pot...
Questions

Mathematics, 20.09.2019 05:10

Computers and Technology, 20.09.2019 05:10

Social Studies, 20.09.2019 05:10

Chemistry, 20.09.2019 05:10






Mathematics, 20.09.2019 05:10


Chemistry, 20.09.2019 05:10







Computers and Technology, 20.09.2019 05:10

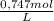 = 0,0237 mol of HCl
= 0,0237 mol of HCl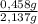 ×100 = 21,4 wt%
×100 = 21,4 wt%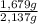 ×100 = 78,6 wt%
×100 = 78,6 wt%

