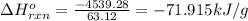Pentaborane−9 (b5h9) is a colorless, highly reactive liquid that will burst into flames when exposed to oxygen. the reaction is 2b5h9(l) + 12o2(g) → 5b2o3(s) + 9h2o(l) calculate the kilojoules of heat released per gram of the compound reacted with oxygen. the standard enthalpy of formations of b5h9(l), b2o3(s), and h2o(l) are 73.2, −1271.94, and −285.83 kj/mol, respectively.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 02:40
For a patient with the following pes statement and interventions, which would be the most appropriate monitoring and evaluating data? pes statement: inadequate calcium intake related to food and nutrition related knowledge deficit as evidenced by statements that the only dietary source of calcium is milk and she believes that she is lactose intolerant. patient’s nutrition prescription is for a diet providing 1200 mg calcium per day. patient was provided with in-depth nutrition education on alternative dietary and supplement sources of calcium. a. calcium intake (at subsequent visit) b. knowledge assessment by asking patient to identify food sources from menus and shopping list (at the end of the current visit) c. serum calcium (at next visit) d. both a and b e. both a and c
Answers: 2


Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3
You know the right answer?
Pentaborane−9 (b5h9) is a colorless, highly reactive liquid that will burst into flames when exposed...
Questions



Mathematics, 20.01.2022 18:50





Physics, 20.01.2022 18:50

Mathematics, 20.01.2022 19:00

Mathematics, 20.01.2022 19:00

Social Studies, 20.01.2022 19:00


Mathematics, 20.01.2022 19:00


Mathematics, 20.01.2022 19:00






![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0352/3055/45485.png)
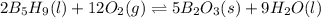
![\Delta H^o_{rxn}=[(n_{(B_2O_3)}\times \Delta H^o_f_{(B_2O_3)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})]-[(n_{(B_5H_9)}\times \Delta H^o_f_{(B_5H_9)})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0352/3055/0889c.png)
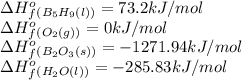
![\Delta H^o_{rxn}=[(5\times -1271.94)+(9\times -285.83)]-[(2\times 73.2)+(12\times 0)]=-9078.57kJ/mol](/tpl/images/0352/3055/ac167.png)
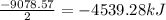
 = 63.12 g/mole
= 63.12 g/mole