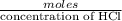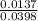Milk of magnesia is often taken to reduce the discomfort associated with acid stomach or heartburn. the recommended dose is 1 teaspoon, which contains 4.00×10²mg of mg(oh)². what volume of an hcl solution with a ph of 1.4 can be neutralized by one dose of milk of magnesia?

Answers: 2


Another question on Chemistry



Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2

Chemistry, 23.06.2019 08:50
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas.caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible.relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3
You know the right answer?
Milk of magnesia is often taken to reduce the discomfort associated with acid stomach or heartburn....
Questions



English, 04.02.2021 01:00




Mathematics, 04.02.2021 01:00

Mathematics, 04.02.2021 01:00


Chemistry, 04.02.2021 01:00



Mathematics, 04.02.2021 01:00

Advanced Placement (AP), 04.02.2021 01:00





Social Studies, 04.02.2021 01:00

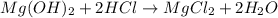
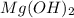 is 58.31 g/mol.
is 58.31 g/mol.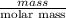
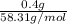
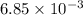 mol
mol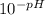 =
= 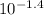 = 0.0398 M
= 0.0398 M