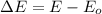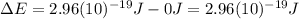Chemistry, 30.10.2019 22:31 swaggernas
A) a red laser pointer emits light with a wavelength of 670 nm. what is the frequency of this light?
b) what is the energy of 1 mole of these photons?
c) the laser pointer emits light because electrons in the material are excited (by a battery) from their ground state to an upper excited state. when the electrons return to the ground state they lose the excess energy in the form of 670 nm photons. what is the energy gap between the ground state and excited state in the laser material?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2
You know the right answer?
A) a red laser pointer emits light with a wavelength of 670 nm. what is the frequency of this light?...
Questions




Mathematics, 11.12.2021 04:30

Mathematics, 11.12.2021 04:30



Mathematics, 11.12.2021 04:30


Social Studies, 11.12.2021 04:30

Computers and Technology, 11.12.2021 04:30


Mathematics, 11.12.2021 04:30

Chemistry, 11.12.2021 04:30



Biology, 11.12.2021 04:30

Mathematics, 11.12.2021 04:30

Mathematics, 11.12.2021 04:30

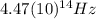
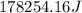
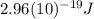
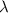 and the frequency
and the frequency  of the light:
of the light:
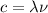 (1)
(1)
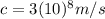 is the speed of light in vacuum
is the speed of light in vacuum
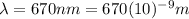 is the wavelength of the light emitted by the laser pointer
is the wavelength of the light emitted by the laser pointer
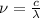 (2)
(2)
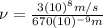 (3)
(3)
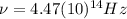 (4) This is the frequency
(4) This is the frequency
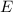 of a 670 nm photon is given by:
of a 670 nm photon is given by:
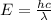 (5)
(5)
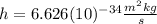 is the Planck constant
is the Planck constant 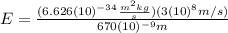 (6)
(6)
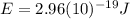 (7) This is the energy of one photon
(7) This is the energy of one photon
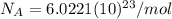 ):
):
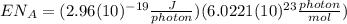 (8)
(8)
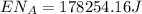 This is the energy of 1 mole of 670 nm photons
This is the energy of 1 mole of 670 nm photons
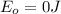 and the energy gap
and the energy gap 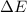 is:
is: