
Chemistry, 31.10.2019 01:31 CaraRose1887
Consider the reaction: 2 so2(g)+o2(g)→2 so3(g) if 285.5 ml of so2 reacts with 158.9 ml of o2 (both measured at 315 k and 50.0 mmhg), what is the limiting reactant and the theoretical yield of so3? if 187.2 ml of so3 is collected (measured at 315 k and 50.0 mmhg), what is the percent yield for the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
Consider the reaction: 2 so2(g)+o2(g)→2 so3(g) if 285.5 ml of so2 reacts with 158.9 ml of o2 (both...
Questions

Mathematics, 28.08.2019 05:50




History, 28.08.2019 05:50

Mathematics, 28.08.2019 05:50

Mathematics, 28.08.2019 05:50

Social Studies, 28.08.2019 05:50



Mathematics, 28.08.2019 05:50



English, 28.08.2019 05:50

Social Studies, 28.08.2019 05:50

Computers and Technology, 28.08.2019 05:50

French, 28.08.2019 05:50



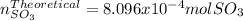
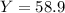 %
%
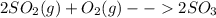
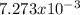 moles of
moles of 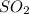 as follows:
as follows: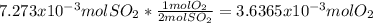
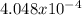 moles are available in comparison with the
moles are available in comparison with the 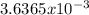 moles that completely would react with
moles that completely would react with 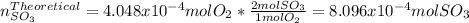
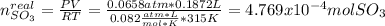
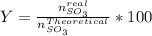 %
%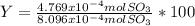 %
%

