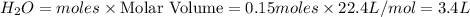Hydrogen chloride gas and oxygen react to form water vapor and chlorine gas. what volume of water would be produced by this reaction if 6.5 l of hydrogen chloride were consumed? also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Calculate the change in entropy if br2(l) is converted to br2(g). s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 3

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
Hydrogen chloride gas and oxygen react to form water vapor and chlorine gas. what volume of water wo...
Questions






Biology, 08.10.2019 04:10

Mathematics, 08.10.2019 04:10




Biology, 08.10.2019 04:10

Arts, 08.10.2019 04:10


English, 08.10.2019 04:10

History, 08.10.2019 04:10


Social Studies, 08.10.2019 04:10


History, 08.10.2019 04:10

History, 08.10.2019 04:10

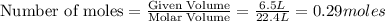

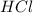 produce = 2 moles of
produce = 2 moles of 
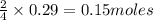 of
of