
Chemistry, 01.11.2019 02:31 santileiva123199
Barium can be analyzed by precipitating it as baso4 and determining the mass of the precipitate. when a 0.269 g sample of a barium compound was treated with excess h2so4, 0.0891 g of baso4 formed. what percentage of barium is in the compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1
You know the right answer?
Barium can be analyzed by precipitating it as baso4 and determining the mass of the precipitate. whe...
Questions

English, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01



History, 11.10.2020 14:01



Computers and Technology, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01



English, 11.10.2020 14:01

English, 11.10.2020 14:01

Arts, 11.10.2020 14:01

Biology, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01


Mathematics, 11.10.2020 14:01

Mathematics, 11.10.2020 14:01

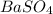 , 1 molecule of
, 1 molecule of 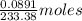 of
of 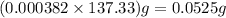
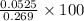 % = 19.5%
% = 19.5%

