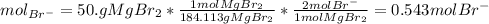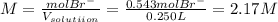Achemist prepares a solution of magnesium bromide by measuring out of into a volumetric flask and filling to the mark with distilled water. calculate the molarity of anions in the chemist's solution. be sure your answer is rounded to the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3
You know the right answer?
Achemist prepares a solution of magnesium bromide by measuring out of into a volumetric flask and fi...
Questions

Mathematics, 12.08.2020 07:01



Health, 12.08.2020 07:01




Social Studies, 12.08.2020 07:01







Biology, 12.08.2020 07:01


Mathematics, 12.08.2020 07:01


Mathematics, 12.08.2020 07:01

Mathematics, 12.08.2020 07:01

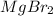 and the volume of the volumetric flask are not given, one can assume arbitrary values and you could modify them whenever you want, thus, let 50.0g of magnesium bromide to be the measured out amount of solute and a 250-mL volumetric flask where the solution is prepared, so the final volume of the solution is 250 mL after the addition of distilled water. In this manner, the bromide anions moles, taking into account there are two bromide moles per magnesium bromide moles, turns out into:
and the volume of the volumetric flask are not given, one can assume arbitrary values and you could modify them whenever you want, thus, let 50.0g of magnesium bromide to be the measured out amount of solute and a 250-mL volumetric flask where the solution is prepared, so the final volume of the solution is 250 mL after the addition of distilled water. In this manner, the bromide anions moles, taking into account there are two bromide moles per magnesium bromide moles, turns out into: