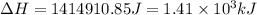Calculate the amount of energy in kilojoules needed to change 459 g of water ice at −10 ∘c to steam at 125 ∘c. the following constants may be useful: cm (ice)=36.57 j/(mol⋅∘c) cm (water)=75.40 j/(mol⋅∘c) cm (steam)=36.04 j/(mol⋅∘c) δhfus=+6.01 kj/mol δhvap=+40.67 kj/mol express your answer with the appropriate units. view available hint(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1


Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
Calculate the amount of energy in kilojoules needed to change 459 g of water ice at −10 ∘c to steam...
Questions


Biology, 01.04.2020 21:36

English, 01.04.2020 21:36

Mathematics, 01.04.2020 21:36


Mathematics, 01.04.2020 21:36




Chemistry, 01.04.2020 21:36


Social Studies, 01.04.2020 21:36

Arts, 01.04.2020 21:36




Mathematics, 01.04.2020 21:37


Mathematics, 01.04.2020 21:37

Mathematics, 01.04.2020 21:37

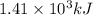
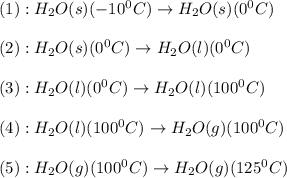
![\Delta H=[n\times c_{ice}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[n\times c_{water}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[n\times c_{steam}\times (T_{final}-T_{initial})]](/tpl/images/0355/0743/9dcae.png)
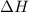 = enthalpy change = ?
= enthalpy change = ?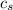 = specific heat of ice =
= specific heat of ice = 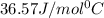
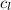 = specific heat of water =
= specific heat of water = 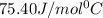
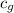 = specific heat of steam =
= specific heat of steam = 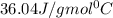
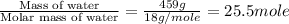
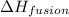 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole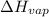 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[25.5mole\times 36.57J/mol^0C\times (0-(-10))^0C]+25.5mole\times 6010J/mole+[25.5mole\times 75.40J/mol^0C\times (100-0)^0C]+25.5mole\times 40670J/mole+[25.5mole\times 36.04J/gmol^0C\times (125-100)^0c]](/tpl/images/0355/0743/cfaa1.png)