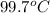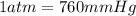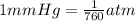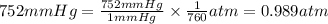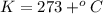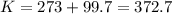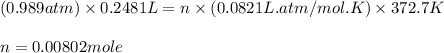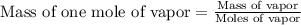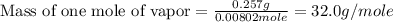Chemistry, 01.11.2019 02:31 katherinevandehei
Astudent weighs an empty flask and stopper and finds the mass to be 55.844 g. she then adds about 5 ml of an unknown liquid and heats the flask in a boiling water bath at 99.7 degrees c. after all the liquid is vaporized, she removes the flask from the bath, stoppers it, and lets it i s cool. after it is cool, she momentarily removes the stopper, then replaces it and weighs the flask and condensed vapor, obtaining a mass of 56.101 g. the volume of the flask is known to be 248.1 ml. the barometric pressure in the laboratory 752 mm. hg. a. what was the pressure of the vapor in the flask atm? p = amt b. what was the temperature of the vapor in k? the volume of the flask in liters? t =, v= l c. what was the mass of vapor that was present in the flask? g = d. how many moles of vapor are present? n = e. what is the mass of one mole of vapor? mm=/mole

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
Astudent weighs an empty flask and stopper and finds the mass to be 55.844 g. she then adds about 5...
Questions



English, 19.10.2019 01:30

Mathematics, 19.10.2019 01:30


Biology, 19.10.2019 01:30




Health, 19.10.2019 01:30



History, 19.10.2019 01:30

English, 19.10.2019 01:30

Mathematics, 19.10.2019 01:30


Health, 19.10.2019 01:30