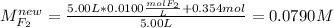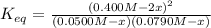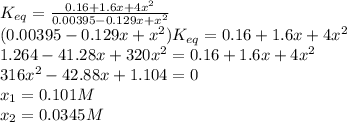Chemistry, 01.11.2019 02:31 krandall232
For the reaction below at a certain temperature, it is found that the equilibrium concentrations in a 5.00 l rigid container are [h2] = 0.0500 m, [f2] = 0.0100 m, and [hf] = 0.400 m. if 0.345 mol of f2 is added to this equilibrium mixture, calculate the concentrations of all gases once equilibrium is reestablished in moles/liter. h2(g) + f2(g) < > 2 hf(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2


Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1
You know the right answer?
For the reaction below at a certain temperature, it is found that the equilibrium concentrations in...
Questions



Mathematics, 08.12.2019 01:31

Social Studies, 08.12.2019 01:31

Mathematics, 08.12.2019 01:31


Mathematics, 08.12.2019 01:31





Biology, 08.12.2019 01:31








![[HF]_{eq}=0.469M, [H_2]_{eq}=0.0155M, [F_2]_{eq}=0.0445M](/tpl/images/0355/0697/87639.png)
![K_{eq}=\frac{[HF]^2}{[H_2][F_2]}=\frac{(0.400M)^2}{(0.0500M)(0.0100M)} =320](/tpl/images/0355/0697/ba1f2.png)