
Chemistry, 01.11.2019 02:31 alexisgilford
Asolid was found to be highly soluble in water. treating separate portions of a solution of this solid with aqueous solutions of nacl, nabr, nai, na2so4, na2co3, na3po4, naoh, and na2s produced no precipitates. treatment of an aqueous solution of this solid with acid resulted in the formation of an odorless gas. this solid could be: a. pbso3b. kbrc. li2co3d. (nh4)2se. both pbso3 and li2co3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1
You know the right answer?
Asolid was found to be highly soluble in water. treating separate portions of a solution of this sol...
Questions

Geography, 14.05.2021 01:00

Mathematics, 14.05.2021 01:00

Health, 14.05.2021 01:00

Mathematics, 14.05.2021 01:00

Mathematics, 14.05.2021 01:00

Mathematics, 14.05.2021 01:00





Chemistry, 14.05.2021 01:00

Mathematics, 14.05.2021 01:00





Mathematics, 14.05.2021 01:00


Mathematics, 14.05.2021 01:00

Mathematics, 14.05.2021 01:00

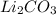
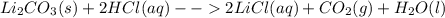
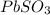 releases
releases  it isn't the solid since that gas is odorous.
it isn't the solid since that gas is odorous.


