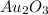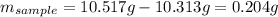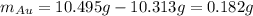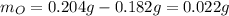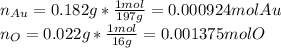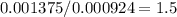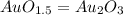Acompound is known to contain only gold and oxygen. a sample of this compound is placed in a clean crucible that has a mass of 10.313 g. the crucible and sample have a mass of 10.517 g. the crucible is heated until the compound decomposes to the elements. the oxygen is lost to the air and the gold remains in the crucible. the mass of the crucible and gold is 10.495 g. what is the empirical formula of this compound?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Acompound is known to contain only gold and oxygen. a sample of this compound is placed in a clean c...
Questions



Mathematics, 25.01.2022 02:10

Mathematics, 25.01.2022 02:10

Mathematics, 25.01.2022 02:10

Chemistry, 25.01.2022 02:10

Mathematics, 25.01.2022 02:10

Mathematics, 25.01.2022 02:10

Social Studies, 25.01.2022 02:10







Mathematics, 25.01.2022 02:10


Mathematics, 25.01.2022 02:10


Mathematics, 25.01.2022 02:10