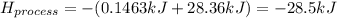Chemistry, 01.11.2019 02:31 tintlemax6256
When 50.0 ml of water containing 0.50 mol hcl at 22.5°c are mixed with 50.0 ml of water containing 0.50 mol naoh at 22.5°c in a calorimeter, the temperature of the solution increases to 26.0°c. how much heat (in kj) was released by this reaction? note: the specific heat of water (cwater) is 4.18 j/(g•˚c) and the density of the solution is 1.00 g/ml.

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 09:10
Complete the following radioactive decay problem. tan+on-? c+th
Answers: 1

Chemistry, 23.06.2019 10:30
Silver is a white metal that is an excellent conductor. silver tarnishes when exposed to air and light. the density of silver is 10.49 g/cm3. the melting point is 962oc and the boiling point is 2000oc. a chemical property of silver is
Answers: 3
You know the right answer?
When 50.0 ml of water containing 0.50 mol hcl at 22.5°c are mixed with 50.0 ml of water containing 0...
Questions

Mathematics, 24.01.2020 00:31

Mathematics, 24.01.2020 00:31

History, 24.01.2020 00:31





Biology, 24.01.2020 00:31

Mathematics, 24.01.2020 00:31

Mathematics, 24.01.2020 00:31




Computers and Technology, 24.01.2020 00:31

Mathematics, 24.01.2020 00:31


Mathematics, 24.01.2020 00:31


Mathematics, 24.01.2020 00:31

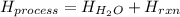
![H_{H_2O}=[(50.0mL+50mL)*\frac{1g}{1mL}]*4.18\frac{J}{mol^0C}*(26.0-22.5)^0C\\H_{H_2O}=146.3J=0.1463kJ](/tpl/images/0355/0596/d18d3.png)