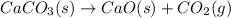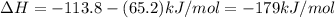Chemistry, 01.11.2019 02:31 blondielocks2002
Determine the enthalpy change for the decomposition of calcium carbonate. caco₃(s) --> cao(s) + co₂(g) given the thermochemical equations below:
ca(oh)₂(s) --> cao(s) + h₂o(l); enthalpy reaction = 65.2 kj/mol
ca(oh)₂(s) + co₂(g) --> caco₃(s) + h₂o(l); enthalpy reaction = -113.8 kj/mol
c(s) + o₂(g) --> co₂(g); enthalpy of reation = -393.5 kj/mol
2ca(s) + o₂(g) --> 2cao(s); enthalpy of reaction = -1270.2 kj/mol
a. 1711.7 kj/mol rxn
b. 441 kj/mol rxn
c. 179 kj/mol rxn
d. 48 kj/mol rxn
e. 345.5 kj. mol rxn

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1


Chemistry, 23.06.2019 00:30
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1
You know the right answer?
Determine the enthalpy change for the decomposition of calcium carbonate. caco₃(s) --> cao(s) +...
Questions


World Languages, 16.09.2019 09:30



Mathematics, 16.09.2019 09:30


Computers and Technology, 16.09.2019 09:30



Biology, 16.09.2019 09:30

Biology, 16.09.2019 09:30

Mathematics, 16.09.2019 09:30

Biology, 16.09.2019 09:30

Mathematics, 16.09.2019 09:30

Mathematics, 16.09.2019 09:30



Mathematics, 16.09.2019 09:30

Mathematics, 16.09.2019 09:30

Mathematics, 16.09.2019 09:30

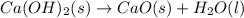
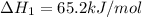 (1)
(1)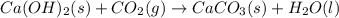
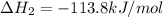 (2)
(2)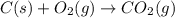
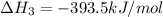 (3)
(3)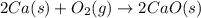
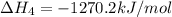 (4)
(4)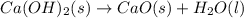 -
-