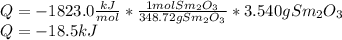A3.540-g sample of an unknown metal m is burned in the presence of excess oxygen, producing the oxide m2o3(s) and liberating 18.56 kj of heat at constant pressure. what is the identity of the metal? 4m(s) + 3o2(g) → 2m2o3(s) substanceδh°f (kj/mol) yb2o3(s)–1814.6 tb2o3(s)–1865.2 sm2o3(s)–1823.0 sc2o3(s)–1908.8 y2o3(s)–1905.3 a) sm b) tb c) y d) sc e) yb

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
A3.540-g sample of an unknown metal m is burned in the presence of excess oxygen, producing the oxid...
Questions

Health, 25.12.2019 19:31




Mathematics, 25.12.2019 19:31

History, 25.12.2019 19:31

History, 25.12.2019 19:31


Advanced Placement (AP), 25.12.2019 19:31

Mathematics, 25.12.2019 19:31

Business, 25.12.2019 19:31

History, 25.12.2019 19:31




Mathematics, 25.12.2019 19:31

Mathematics, 25.12.2019 19:31



Mathematics, 25.12.2019 19:31