
If the aluminum block is initially at 25 ∘c, what is the final temperature of the block after the evaporation of the alcohol? assume that the heat required for the vaporization of the alcohol comes only from the aluminum block and that the alcohol vaporizes at 25 ∘c. the heat of vaporization of the alcohol at 25 ∘c is 45.4 kj/mol, the specific heat of aluminum is 0.903 j/g⋅∘c

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
If the aluminum block is initially at 25 ∘c, what is the final temperature of the block after the ev...
Questions



Mathematics, 03.10.2019 01:00








Mathematics, 03.10.2019 01:00


Mathematics, 03.10.2019 01:00







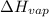 ) = 45.4 kJ/mol
) = 45.4 kJ/mol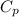 ) = 0.903
) = 0.903 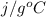
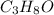 and its mass is 1.12 g. Also, mass of aluminium block is 73.0 g.
and its mass is 1.12 g. Also, mass of aluminium block is 73.0 g.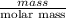
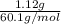
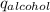 ) = heat lost by aluminium (
) = heat lost by aluminium (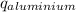 )
)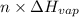 =
= 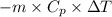
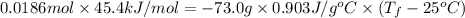
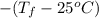
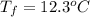
 .
.
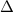 H)= 45.04 kJ/mol
H)= 45.04 kJ/mol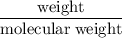
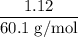
 45.04 kJ/mol
45.04 kJ/mol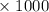 J = - 73
J = - 73 


