
Chemistry, 18.09.2019 07:30 Lilbre9444
Asample of a compound contains 65.4 grams of zinc, 12.0 grams of carbon, and 48.0 grams of oxygen. what is the mole ratio of zinc to carbon
to oxygen in this compound?
(1) 1: 1: 2 (3) 1: 4: 6
(2) 1: 1: 3 (4) 5: 1: 4

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
Asample of a compound contains 65.4 grams of zinc, 12.0 grams of carbon, and 48.0 grams of oxygen. w...
Questions


Mathematics, 09.12.2020 02:20

Mathematics, 09.12.2020 02:20





Mathematics, 09.12.2020 02:20


Mathematics, 09.12.2020 02:20









Mathematics, 09.12.2020 02:20

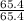 = 1
= 1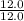 = 1
= 1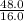 = 3
= 3

