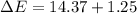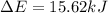Chemistry, 02.11.2019 03:31 Jsanders2276
In a piston, the addition of 14.37 kj of heat to a 100. g sample of a liquid at a constant temperature of 35.2 °c caused the liquid to vaporize (change to a gas). the vaporized gas expanded against an external pressure of 1.07 atm and a volume change of 11.49 l was observed. (recall: 1 l• atm = 101.3 j} what was the change in the internal energy of the system, (ae in kj)? (enter your answer with two decimals places and no units.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1
You know the right answer?
In a piston, the addition of 14.37 kj of heat to a 100. g sample of a liquid at a constant temperatu...
Questions

Biology, 09.09.2021 20:40


Mathematics, 09.09.2021 20:40

Social Studies, 09.09.2021 20:40






English, 09.09.2021 20:40

Health, 09.09.2021 20:40





Physics, 09.09.2021 20:40



Mathematics, 09.09.2021 20:40

Mathematics, 09.09.2021 20:40


 = change in volume = 11.49 L
= change in volume = 11.49 L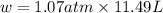
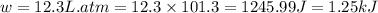 (as per conversion)
(as per conversion)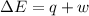
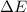 = internal energy of the system
= internal energy of the system