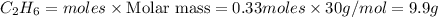The theoretical yield of a reaction is the amount of product obtained if the limiting reactant is completely converted to product. consider the reaction: h2(g) + c2h4(g) → c2h6(g) if 8.150 g h2 is mixed with 9.330 g c2h4, calculate the theoretical yield (g) of c2h6 produced by the reaction.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2


Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
The theoretical yield of a reaction is the amount of product obtained if the limiting reactant is co...
Questions


Mathematics, 03.03.2021 23:30






History, 03.03.2021 23:30







English, 03.03.2021 23:30

Mathematics, 03.03.2021 23:30

Mathematics, 03.03.2021 23:30


Mathematics, 03.03.2021 23:30

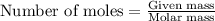
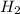
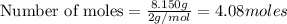
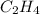
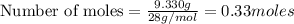
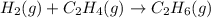
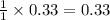 mole of
mole of 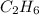
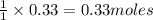 of
of