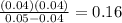Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Trichloroacetic acid (ccl3co2h) is a corrosive acid that is used to precipitate proteins. the ph of...
Questions

Mathematics, 27.06.2019 07:30




Mathematics, 27.06.2019 07:30



History, 27.06.2019 07:30


Physics, 27.06.2019 07:30

History, 27.06.2019 07:30

Mathematics, 27.06.2019 07:30





History, 27.06.2019 07:30



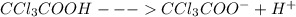
![\frac{[H^{+}][ClO_{4}^{-}]}{[HClO_{4}]}](/tpl/images/0356/4769/5addd.png)