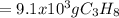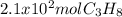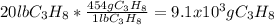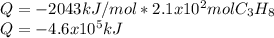How many grams of propane are in 20 pounds of propane? use the conversion 1 lb = 454 g. (express your answers for the next three questions in scientific notation. for example use 2.3e-5 to indicate a number such as 2.3 x 10-5.) 9.1e3 grams b) how many moles of propane are in 20 pounds of propane? 2.1e2 moles c)how much heat can be obtained by burning 20 pounds of propane? (remember to look at this from the viewpoint of the surroundings, since the question asks how much heat can be obtained.) 4.6e5 kj

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1
You know the right answer?
How many grams of propane are in 20 pounds of propane? use the conversion 1 lb = 454 g. (express yo...
Questions





Mathematics, 14.04.2020 01:54

Mathematics, 14.04.2020 01:54

Mathematics, 14.04.2020 01:54

History, 14.04.2020 01:54

Chemistry, 14.04.2020 01:54


Mathematics, 14.04.2020 01:54

Mathematics, 14.04.2020 01:54







Computers and Technology, 14.04.2020 01:57

Mathematics, 14.04.2020 01:57