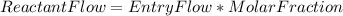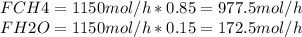Chemistry, 02.11.2019 04:31 Svetakotok
Steam reforming of natural gas is the most common method of producing commercial hydrogen. a stream with a flow rate of 1150 mol/h containing 85.0 mol% ch4 and 15.0 mol% of water is combined with additional water steam and fed to a steam reforming reactor to produce hydrogen. the stream coming out is in chemical equilibrium. the fractional conversion for both the water and methane are 0.600. balance the chemical equation and calculate how much additional water steam is fed to the steam reforming reactor and the flow rate of the outlet hydrogen. ch4 + h2o < --> co + 3h2 (balanced)

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2

Chemistry, 23.06.2019 00:30
Which of the following best describes technology a. something created for only scientists to use b.the method of thinking that scientists use. c.the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
Steam reforming of natural gas is the most common method of producing commercial hydrogen. a stream...
Questions


Mathematics, 08.12.2020 03:30


Mathematics, 08.12.2020 03:30

Mathematics, 08.12.2020 03:30




History, 08.12.2020 03:30

Social Studies, 08.12.2020 03:30

Mathematics, 08.12.2020 03:30


Mathematics, 08.12.2020 03:30

Mathematics, 08.12.2020 03:30


Arts, 08.12.2020 03:30


Arts, 08.12.2020 03:30

History, 08.12.2020 03:30

History, 08.12.2020 03:30