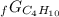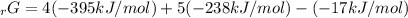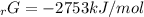Chemistry, 02.11.2019 05:31 WhiteMex69
Given the values of δgfo given below in kj/mol, calculate the value of δgo in kj for the combustion of 1 mole of butane to form carbon dioxide and liquid water. δgfo (c4h10(g)) = -17 δgfo (co2(g)) = -395 δgfo (h2o(l)) = -238

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1


Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
Given the values of δgfo given below in kj/mol, calculate the value of δgo in kj for the combustion...
Questions

Mathematics, 16.08.2019 21:10



Mathematics, 16.08.2019 21:10

Mathematics, 16.08.2019 21:10




Social Studies, 16.08.2019 21:10

History, 16.08.2019 21:10



Mathematics, 16.08.2019 21:10




Physics, 16.08.2019 21:10

Mathematics, 16.08.2019 21:10



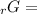 4Δ
4Δ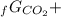 5Δ
5Δ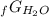 -Δ
-Δ