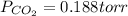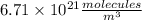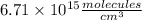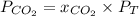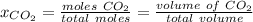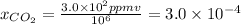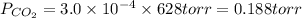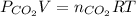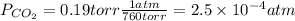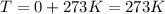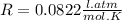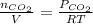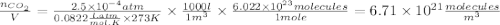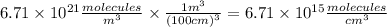Chemistry, 02.11.2019 05:31 jadeafrias
Atmospheric scientists often use mixing ratios to express the concentrations of trace compounds in air. mixing ratios are often expressed as ppmv (parts per million volume): ppmv of x 5 vol of x at stp 3 106 total vol of air at stp on a certain november day, the concentration of carbon monoxide in the air in downtown denver, colorado, reached 3.0 3 102 ppmv. the atmospheric pressure at that time was 628 torr and the temperature was 08c.
a. what was the partial pressure of co?
b. what was the concentration of co in molecules per cubic meter?
c. what was the concentration of co in molecules per cubic centimeter?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:50
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 07:20
The diagrams show objects’ gravitational pull toward each other. which statement describes the relationship between diagram x and y? gravity attracts only larger objects toward one another. gravity attracts larger objects only if they are close to one another. if the masses of the objects increase, then the force between them also increases. if distance between the objects increases, then the amount of force also increases.
Answers: 1

Chemistry, 22.06.2019 08:00
An observation that requires measurement is called quantitative observable or qualitative
Answers: 1
You know the right answer?
Atmospheric scientists often use mixing ratios to express the concentrations of trace compounds in a...
Questions


Computers and Technology, 18.12.2019 01:31


Mathematics, 18.12.2019 01:31





Spanish, 18.12.2019 01:31

Mathematics, 18.12.2019 01:31

Mathematics, 18.12.2019 01:31


Biology, 18.12.2019 01:31

History, 18.12.2019 01:31


History, 18.12.2019 01:31

Mathematics, 18.12.2019 01:31

Mathematics, 18.12.2019 01:31


Mathematics, 18.12.2019 01:31