
Chemistry, 02.11.2019 05:31 itsme123427
Aprotein was previously determined to contain 15.7 wt% nitrogen. a 647 m aliquot of a solution containing the protein was digested in boiling sulfuric acid. the solution was made basic and the liberated nh3 was collected in 10.00 ml of 0.0388 m hci. 3.83 ml of 0.0196 m naoh was required to react with the excess, unreacted hcl. calculate the protein concentration of the solution in units of milligrams per milliliter. number 0 mg protein ml solution

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
Aprotein was previously determined to contain 15.7 wt% nitrogen. a 647 m aliquot of a solution conta...
Questions

Mathematics, 08.03.2021 20:00



Mathematics, 08.03.2021 20:00

Computers and Technology, 08.03.2021 20:00


Computers and Technology, 08.03.2021 20:00







Computers and Technology, 08.03.2021 20:00



Mathematics, 08.03.2021 20:00



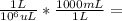 0.647 mL27.91 mg / 0.647 mL = 431.38 mg/mL
0.647 mL27.91 mg / 0.647 mL = 431.38 mg/mL

