
Chemistry, 02.11.2019 06:31 xxbloomxx184p8jqjd
2.122 g of a solid mixture containing only potassium carbonate (fw = 138.2058 g/mol) and potassium bicarbonate (fw = 100.1154 g/mol) is dissolved in distilled water. 34.16 ml of a 0.762 m hcl standard solution is required to titrate the mixture to a bromocresol green end point. calculate the weight percent of potassium carbonate and potassium bicarbonate in the mixture.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
2.122 g of a solid mixture containing only potassium carbonate (fw = 138.2058 g/mol) and potassium b...
Questions



Mathematics, 06.11.2020 18:00

Mathematics, 06.11.2020 18:00

Biology, 06.11.2020 18:00






Mathematics, 06.11.2020 18:00



History, 06.11.2020 18:00

Mathematics, 06.11.2020 18:00


Health, 06.11.2020 18:00

Mathematics, 06.11.2020 18:00

Business, 06.11.2020 18:00

Mathematics, 06.11.2020 18:00

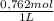 = 0,02603 mol of HCl
= 0,02603 mol of HCl + Y×
+ Y×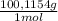 (1)
(1)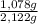 ×100 = 50,8 wt%
×100 = 50,8 wt%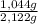 ×100 = 49,2 wt%
×100 = 49,2 wt%

