
Chemistry, 02.11.2019 07:31 morganhines181
Solution of the schrödinger wave equation for the hydrogen atom results in a set of functions (orbitals) that describe the behavior of the electron. each function is characterized by 3 quantum numbers: n, l, and ml. if the value of n = 1 the quantum number l can have values from to . the total number of orbitals possible at the n = 1 energy level is . if the value of l = 1 the quantum number ml can have values from to . the total number of orbitals possible at the l = 1 sublevel is .

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
Solution of the schrödinger wave equation for the hydrogen atom results in a set of functions (orbit...
Questions



History, 07.01.2022 20:10

English, 07.01.2022 20:10

Mathematics, 07.01.2022 20:10

Mathematics, 07.01.2022 20:10

History, 07.01.2022 20:10




Social Studies, 07.01.2022 20:10

Mathematics, 07.01.2022 20:10

English, 07.01.2022 20:10

Mathematics, 07.01.2022 20:10

Mathematics, 07.01.2022 20:10




Mathematics, 07.01.2022 20:20

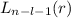 , being n and l the quantum numbers. As is known, in the Laguerre polynomials the subindex must be greater than or equal to 0, which implies
, being n and l the quantum numbers. As is known, in the Laguerre polynomials the subindex must be greater than or equal to 0, which implies 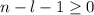 . So if n=1, the only possible value of l is l=0.
. So if n=1, the only possible value of l is l=0. ![Y_{l,m_l}(\theta,\phi)[\tex] as solution. One of the properties of spherical harmonics is that the number [tex]m_l[\tex] can only take the values [tex]m_l=-l,-l+1,-l+2,...,l-1,l[\tex]. So if l=0, as above, [tex]m_l[\tex] can only take the value [tex]m_l=0,[\tex] thus giving only 1 orbital. But if l=1, [tex]m_l](/tpl/images/0356/6953/a304c.png) can be
can be 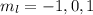 . Therefore the sublevel l=| has 3 possible orbitals.
. Therefore the sublevel l=| has 3 possible orbitals.

