
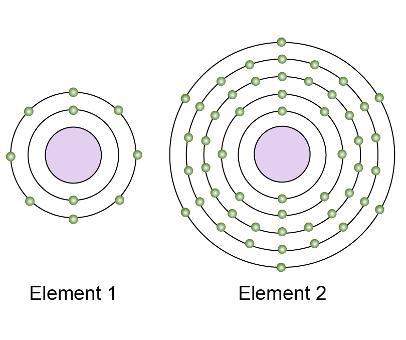
These models show the electron structures of two different nonmetal elements.
which element is likely more reactive, and why?
a.) element 1 is more reactive because it has fewer electron shells and is toward the top of its group on the periodic table.
b.) element 1 is more reactive because it has more electrons in its valence shell and is farther to the right on the periodic table.
c.) element 2 is more reactive because it does not have a valence shell close to the nucleus, so it will attract electrons.
d.) element 2 is more reactive because it does not have a full valence shell, so it will attract electrons.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 23.06.2019 06:50
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1

Chemistry, 23.06.2019 12:40
During an experiment, ice and water were placed in a perfectly insulated thermos flask at 0 °c. describe this system when it phase reaches equilibrium.
Answers: 1

Chemistry, 23.06.2019 13:20
Which nuclide is most likely to be radioactive and synthetic 24/12 mg237/93mg195/78mg230/84mg
Answers: 1
You know the right answer?
These models show the electron structures of two different nonmetal elements.
which elem...
which elem...
Questions

Mathematics, 13.01.2021 16:50





Mathematics, 13.01.2021 16:50

Engineering, 13.01.2021 16:50

Mathematics, 13.01.2021 16:50

English, 13.01.2021 16:50



Mathematics, 13.01.2021 16:50

Advanced Placement (AP), 13.01.2021 16:50

English, 13.01.2021 16:50


English, 13.01.2021 16:50


English, 13.01.2021 16:50


Law, 13.01.2021 16:50



