
Chemistry, 04.11.2019 19:31 carolyntowerskemp
What is the percent yield for this reaction under the given conditions? i think i have the right answer(16.6%), but can someone check it for me? !
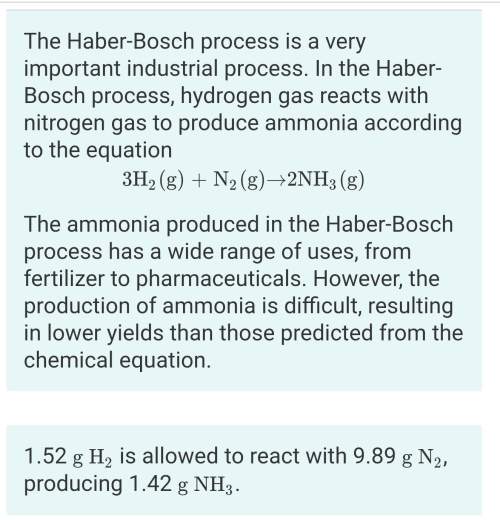

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
You know the right answer?
What is the percent yield for this reaction under the given conditions? i think i have the right an...
Questions


Mathematics, 02.03.2021 06:30

Mathematics, 02.03.2021 06:30


English, 02.03.2021 06:30

Mathematics, 02.03.2021 06:30


Mathematics, 02.03.2021 06:30

Mathematics, 02.03.2021 06:30

Mathematics, 02.03.2021 06:30


Mathematics, 02.03.2021 06:30

Mathematics, 02.03.2021 06:30


Mathematics, 02.03.2021 06:30

Mathematics, 02.03.2021 06:30






