
Chemistry, 04.11.2019 20:31 jakhunter354
Achemical reaction takes place inside a flask submerged in a water bath. the water bath contains 8.10kg of water at 33.9 degrees celsius . during the reaction 69.0kj of heat flows out of the bath and into the flask.
calculate the new temperature of the water bath. you can assume the specific heat capacity of water under these conditions is 4.18j*g*k. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Achemical reaction takes place inside a flask submerged in a water bath. the water bath contains 8.1...
Questions

Mathematics, 03.05.2020 14:26

English, 03.05.2020 14:26


English, 03.05.2020 14:27

Mathematics, 03.05.2020 14:27

Mathematics, 03.05.2020 14:27


Mathematics, 03.05.2020 14:27

Mathematics, 03.05.2020 14:27

Mathematics, 03.05.2020 14:27


Mathematics, 03.05.2020 14:27


Mathematics, 03.05.2020 14:27



Physics, 03.05.2020 14:27

Mathematics, 03.05.2020 14:27


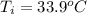 = (33.9 + 273) K = 306.9 K
= (33.9 + 273) K = 306.9 K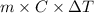
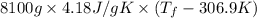
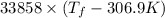
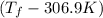 = 2.037 K
= 2.037 K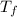 = (2.037 + 306.9) K
= (2.037 + 306.9) K

