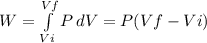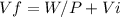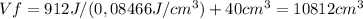Consider a mixture of air and gasoline vapor in a cylinder with a piston. the original volume is 40. cm3. if the combustion of this mixture releases 912 j of energy, to what volume will the gases expand against a constant pressure of 635 torr if all the energy of combustion is converted into work to push back the piston?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
Consider a mixture of air and gasoline vapor in a cylinder with a piston. the original volume is 40....
Questions

Mathematics, 30.10.2020 03:40

Mathematics, 30.10.2020 03:40

Mathematics, 30.10.2020 03:40

Mathematics, 30.10.2020 03:40

Mathematics, 30.10.2020 03:40


History, 30.10.2020 03:40

Mathematics, 30.10.2020 03:40

Biology, 30.10.2020 03:40



Geography, 30.10.2020 03:40


Business, 30.10.2020 03:40

Mathematics, 30.10.2020 03:40



Biology, 30.10.2020 03:40

Chemistry, 30.10.2020 03:40