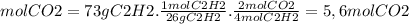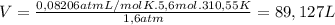Chemistry, 04.11.2019 22:31 kenleighbrooke67
Ethyne gas combusts with oxygen gas according to the following reaction: calculate the volume, in ml of co2 produced when 73 g of c2h2 react at 37.4 °c and 1.6 atm. (r = 0.08206 l atm/mol k) latex: 2\: c_2h_2\left(g\right)\: +\: 5o_2\left(g\right)\: \longrightarrow\: 4\: co_2\left(g\right)\: +2\: h_2o\left(l\right)\:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
Ethyne gas combusts with oxygen gas according to the following reaction: calculate the volume, in m...
Questions




History, 21.07.2019 18:30


Social Studies, 21.07.2019 18:30






Health, 21.07.2019 18:30


Chemistry, 21.07.2019 18:30

Chemistry, 21.07.2019 18:30

World Languages, 21.07.2019 18:30



Social Studies, 21.07.2019 18:30