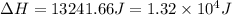Chemistry, 04.11.2019 23:31 mentatmenot
Calculate the enthalpy change (in joules) involved in converting 5.00 grams of water at 14.0 °c to steam at 115 °c under a constant pressure of 1 atm. the specific heats of ice, liquid water, and steam are, respectively, 2.03, 4.18, 1.84 j/g-k and for water δhfusion = 6.01 kj/mole and δhvap = 40.67 kj/mole
a. 1.32x10^4 j
b. 2.05 x 10^5 j
c. 195x 10^3 j
d. 1.94 x 10^3 j

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1


Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
Calculate the enthalpy change (in joules) involved in converting 5.00 grams of water at 14.0 °c to s...
Questions

Mathematics, 15.04.2021 19:30

Mathematics, 15.04.2021 19:30

Physics, 15.04.2021 19:30

Physics, 15.04.2021 19:30

English, 15.04.2021 19:30

Biology, 15.04.2021 19:30

Mathematics, 15.04.2021 19:30



English, 15.04.2021 19:30


Mathematics, 15.04.2021 19:30



Mathematics, 15.04.2021 19:30


Engineering, 15.04.2021 19:30

Mathematics, 15.04.2021 19:30


Mathematics, 15.04.2021 19:30

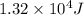
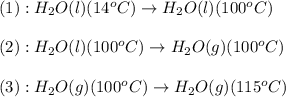
![\Delta H=[m\times c_{p,l}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[m\times c_{p,g}\times (T_{final}-T_{initial})]](/tpl/images/0359/4713/cc64b.png)
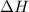 = enthalpy change or heat required = ?
= enthalpy change or heat required = ?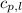 = specific heat of liquid water =
= specific heat of liquid water = 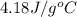
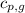 = specific heat of liquid water =
= specific heat of liquid water = 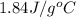
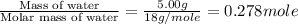
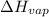 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[5.00g\times 4.18J/g^oC\times (100-14)^oC]+0.278mole\times 40670J/mole+[5.00g\times 1.84J/g^oC\times (115-100)^oC]](/tpl/images/0359/4713/26444.png)