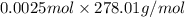Chemistry, 04.11.2019 23:31 pinkycupcakes3oxbqhx
Apowder contains feso4⋅7h2o (molar mass=278.01 g/mol), among other components. a 2.955 g sample of the powder was dissolved in hno3 and heated to convert all iron to fe3+. the addition of nh3 precipitated fe2o3⋅xh2o, which was subsequently ignited to produce 0.201 g fe2o3. what was the mass of feso4⋅7h2o in the 2.955 g sample?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
Apowder contains feso4⋅7h2o (molar mass=278.01 g/mol), among other components. a 2.955 g sample of t...
Questions

Health, 18.02.2021 01:40



Social Studies, 18.02.2021 01:40

Spanish, 18.02.2021 01:40

Mathematics, 18.02.2021 01:40

Computers and Technology, 18.02.2021 01:40


Mathematics, 18.02.2021 01:40


Social Studies, 18.02.2021 01:40


Biology, 18.02.2021 01:40





Mathematics, 18.02.2021 01:40


Mathematics, 18.02.2021 01:40

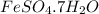 =
= 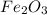
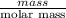
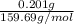
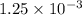 mol
mol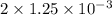 mol
mol